(ITUNES OR LISTEN HERE)
The Free Open Access Medical Education (FOAM)
Dr. Josh Farkas of the PulmCrit blog has produced a couple of blog posts on the importance of renal protection in sepsis, Renoresuscitation: Sepsis resuscitation designed to avoid long-term complications and Renal microvascular hemodynamics in sepsis: a new paradigm. Much of this is theoretical and certainly not something that is standard practice, rathery a theory extrapolated from subgroups of several trials.
Suggested renoresuscitation measures:
(1) Avoid renal failure – avoid nephrotoxins (many antibiotics, NSAIDs, ace-inhibitors), avoid hyperchloremic metabolic acidosis.
(2) Avoid volume overload – treating decreased urine output by flooding a patient with fluids is not necessarily the best move.
(3) Protect the glycocalyx of the endothelium – this suggestion proffers more questions than answers. Steroids? Albumin? Certain vasopressors? Stay tuned, as we’re not really certain what this entails.
The Bread and Butter
We summarize some key topics from Rosenalli, that’s Tintinalli (7e) Chapter 91; Rosen’s (8e) Chapter 97. But, don’t just take our word for it. Go enrich your fundamental understanding yourself.
Acute Kidney Injury – typically a creatinine 1.5-2x the patient’s baseline is classified as acute kidney injury. Urine output can be increased initially but determine whether a patient is making urine and how much, as urine output <0.5 mL/kg/h qualifies as AKI.
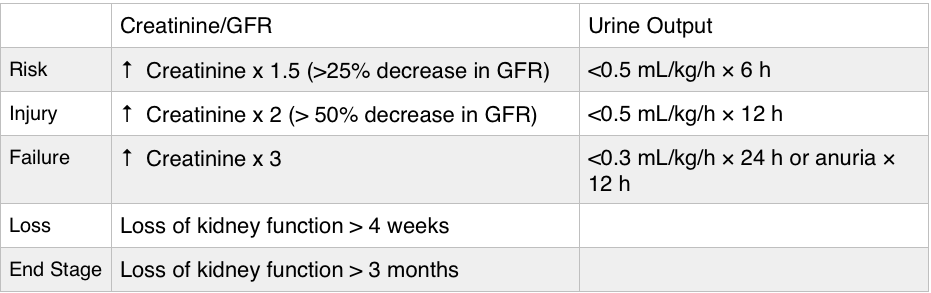
Importance – AKI is associated with worse outcomes, although it’s unclear as to whether this is merely a marker of
- Found in 35-65% of admissions to the intensive care unit, in 5-20% of hospital admissions. Furthermore, AKI is associated with higher mortality.
- Renal failure can also cause significant problems for the patient such as electrolyte abnormalities (hyperkalemia the most worriesome, but also hyperphosphatemia) and pulmonary edema.
Etiology – many causes of AKI are reversible or amenable to treatment.
Prerenal – this is one of the most common causes of acute kidney injury and basically is caused by decreased blood flow to the kidney. Associated with a high BUN/creatinine ratio, increased urine osmolality, a urine sodium concentration less than 20 mEq/L, and FENa less than 1% (this is why getting urine sodium and a concurrent chemistry panel is key).
- Hypovolemia – volume depleted, hemorrhage, intravascular volume depletion from congestive heart failure or cirrhosis.
- Hypotension – poor cardiac output (heart failure, valvular problems), shock
- Decreased flow through the renal artery disease – Nonsteroidal anti-inflammatories: inhibit prostaglandins in the afferent arteriole. ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, leading to decreased levels of angiotensin II, which when absent decreases the GFR because of dilatation of the efferent arteriole.
Post Renal (Obstructive) – Check out Episode 2 on urologic emergencies.
- Benign prostatic hypertrophy (BPH) is the most common cause but medications such as anticholinergics and pseudoephedrine. Trauma, stones, strictures, and malignancy can also cause obstruction.
Intrinsic acute renal failure– divided into: tubular disease (most common), glomerular disease, vascular disease and interstitial disease.
- Least common form of AKI in the ED, more common in inpatients.
- Acute Tubular Necrosis (ATN) most common cause – via nephrotoxins such as aminoglycosides and contrast.
- Granular “muddy brown” casts – think of necrosis from the “N” in ATN and necrosis tends to be dark.
Indications for emergent dialysis – AEIOU
A- Acidosis
E- Electrolyte emergencies (hyperkalemia!)
I- Intoxication with dialyzable toxins (ethylene glycol)
O- Overloaded with volume
U- Uremia
Generously Donated Rosh Review Questions
Question 1. A 72-year-old man is brought to the ED from a nursing home for evaluation of oliguria. He is found to have an acutely elevated BUN and plasma creatinine from baseline. A Foley catheter is placed; his urine sodium (UNa) is measured below 20 mEq/L and fractional excretion of sodium (FENa) below 1%. [polldaddy poll=8545511]
Question 2. A 54-year-old man presents to the ED in acute renal failure (ARF). [polldaddy poll=8545512]
Answer 1. D. This patient’s oliguria with acutely elevated BUN and plasma creatinine suggest that he is in acute renal failure (ARF). His UNa <20 mEq/L and FENa <1% indicate that he has intact reabsorptive function and is able to conserve sodium. This is consistent with prerenal azotemia as the cause for his ARF.
Acute tubular necrosis (ATN) (A), loop diuretics (e.g., furosemide) (B), and osmotic diuresis (e.g., mannitol) (C)all lead to UNa >20 mEq/L and FENa >1% because there is impairment in the ability to concentrate the urine. In such cases, a high-sodium load is excreted.
Answer 2. A. Acute tubular necrosis (ATN) is a severe form of impairment of tubular epithelial cells caused by ischemia or toxic injury. It is a leading cause of ARF. One of its hallmarks is the presence of brown granular casts on urinalysis. These contain cellular debris rich in cytochrome pigments. In contrast, hyaline casts (B) are usually nonspecific but present after exercise; red cell casts (C) are indicative of glomerular hematuria (e.g., glomerulonephritis); and white cell casts (D) imply renal parenchymal inflammation (e.g., acute interstitial nephritis, pyelonephritis).