We are bringing you updates on COVID-19. Note: If you are listening to these more than a few days in the future, please beware that information may have changed and check subsequent episodes. This episode was recorded May 30, 2020
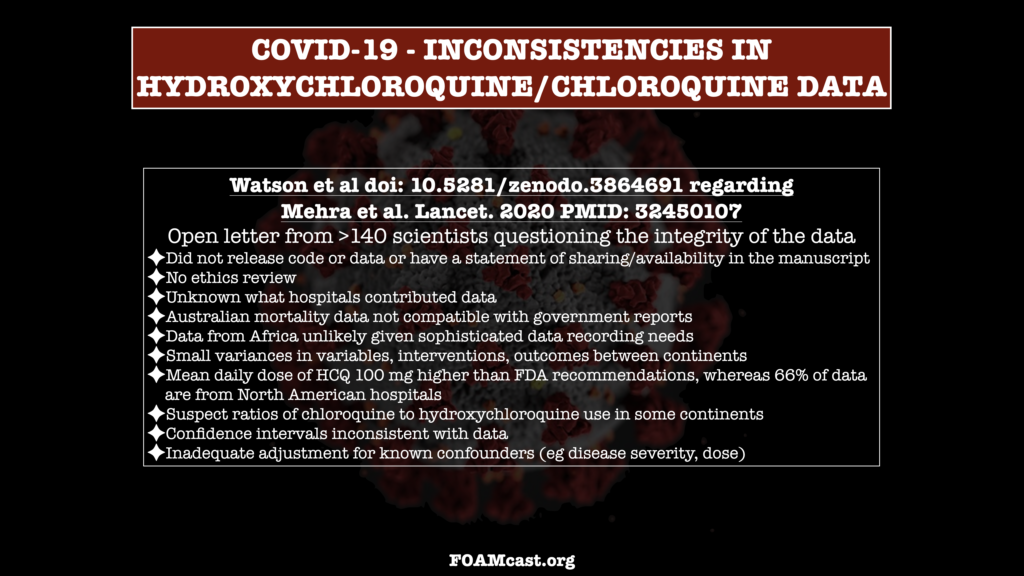
On May 22, the Lancet published a study by Mehra et al that reported an analysis of an enormous international registry of 96,032 patients with COVID-19 that reported patients who received hydroxychloroquine, chloroquine, or these drugs combined with a macrolide had higher risk of mortality. This led to the WHO halting trials. At the same time, remdesivir’s popularity rose and the future for HCQ became bleak. However, some of the numbers just didn’t add up…and, it turns out, it is possible that the data was fabricated or some other serious wrongdoing occurred. More than 100 scientists from around the world have authored an open letter (Watson et al) regarding this concern.

There is one other published paper using the Surgical Outcomes Collaboration, unfortunately, this was the large NEJM study on ACE-I/ARBs in COVID-19, which suffers from similarly improbable data …see this thread

*UPDATE 6/4/20* The authors have issued notices of retraction for both the hydroxychloroquine article as well as the ACE-I/ARB study , citing that they did not have access to the raw data. This is interesting because the NEJM letter is also signed by the author who is the CEO of Surgisphere, who curates the database used in the study, and the corresponding author reported in the original manuscript having full access to the data.
One thought on “COVID-19: Inconsistencies in hydroxychloroquine data in the Lancet”
Comments are closed.