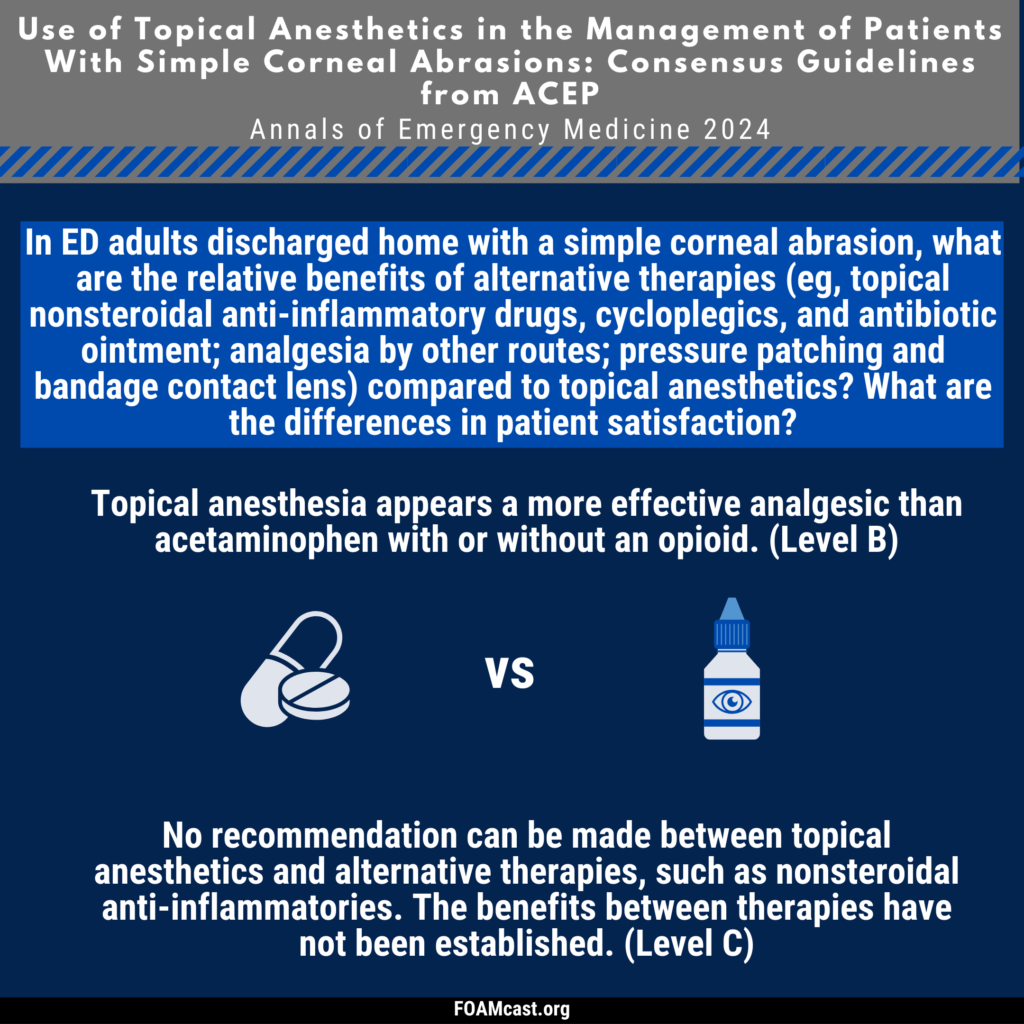
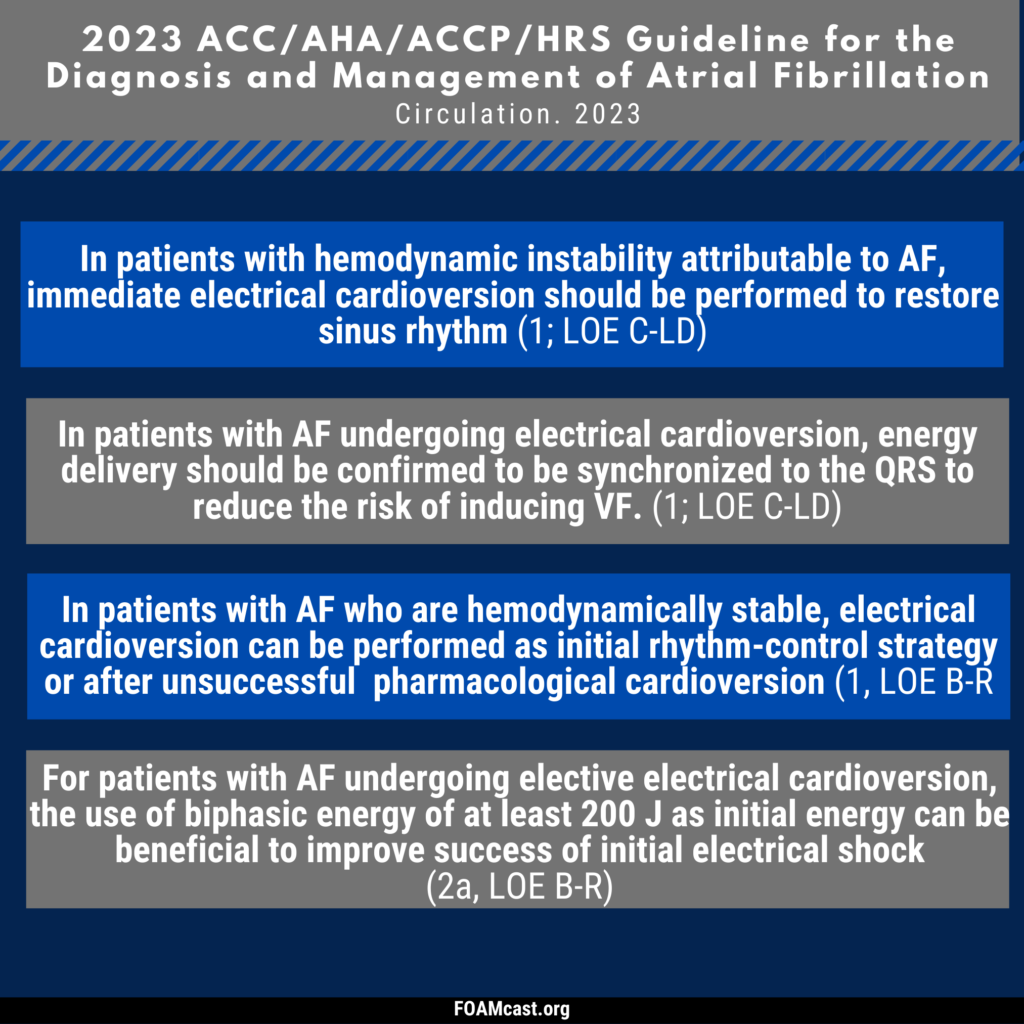
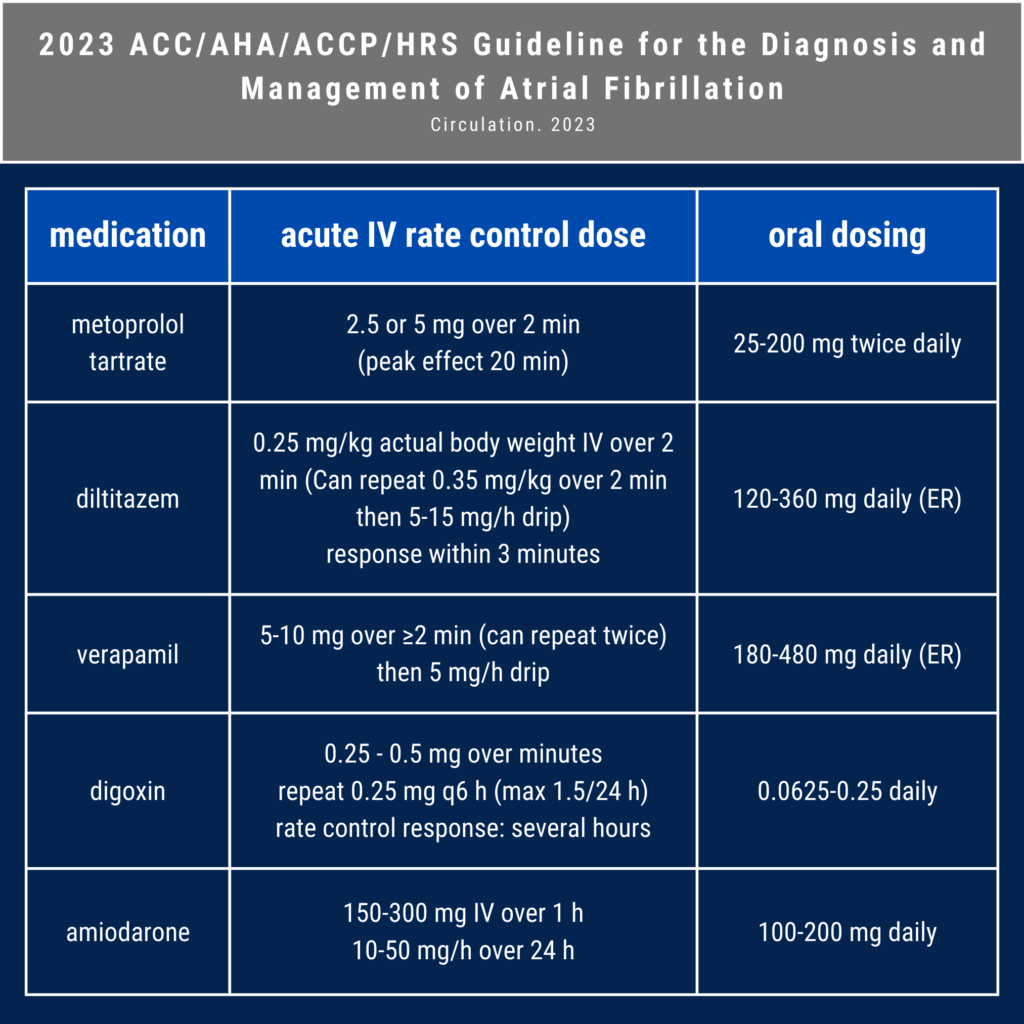
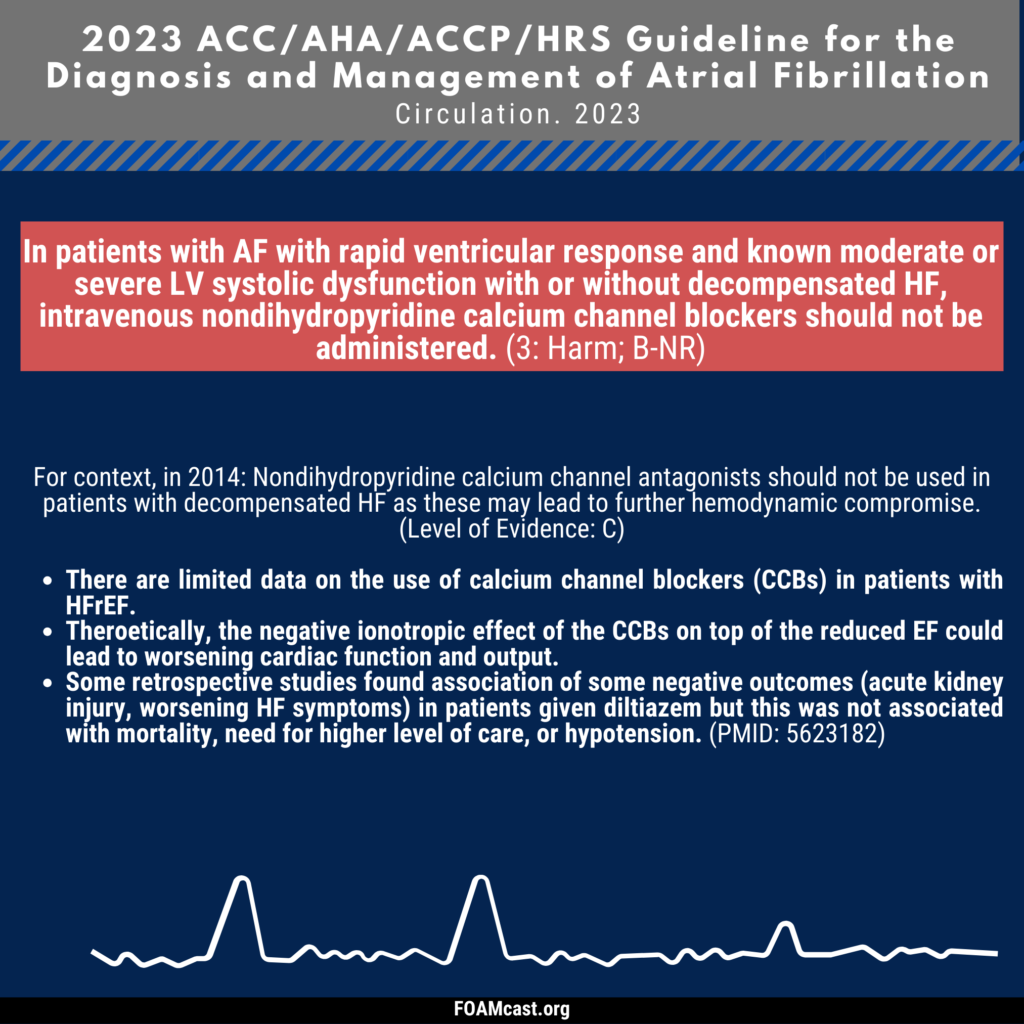
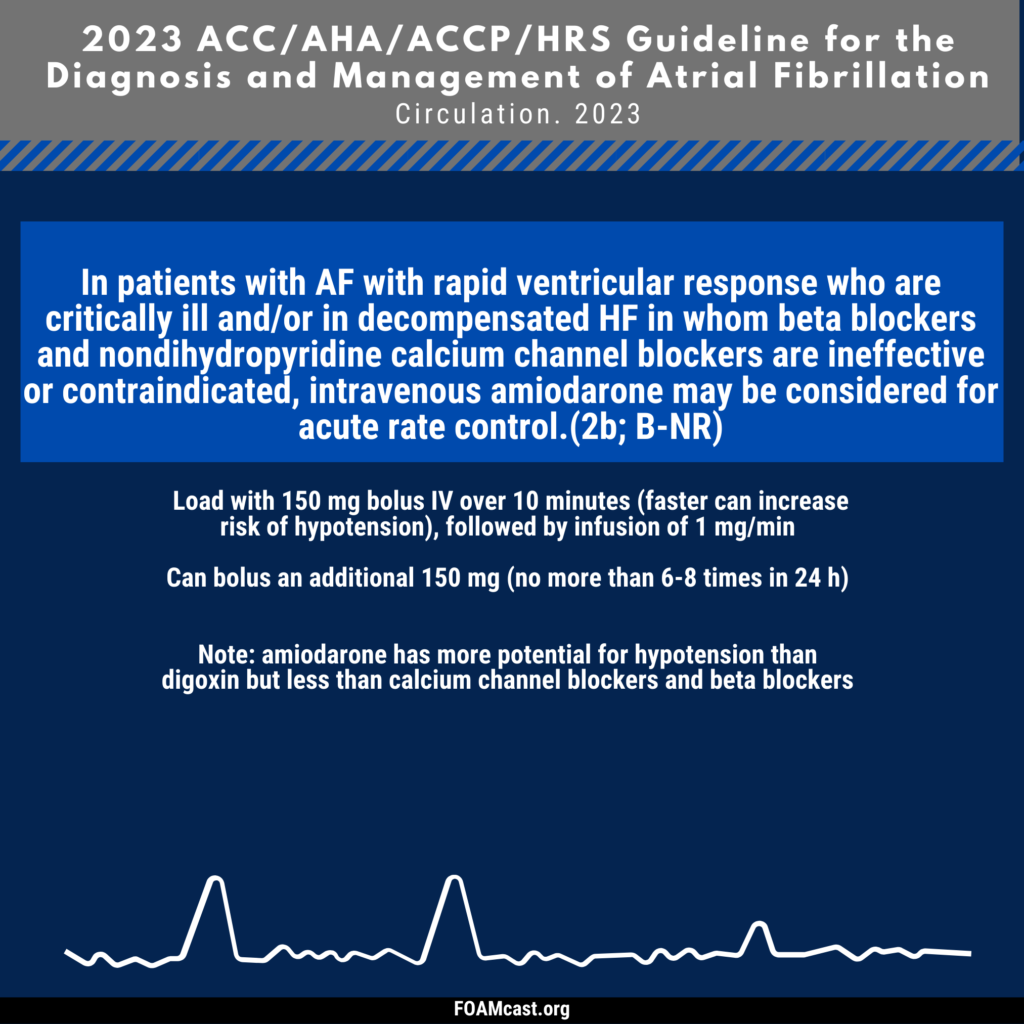
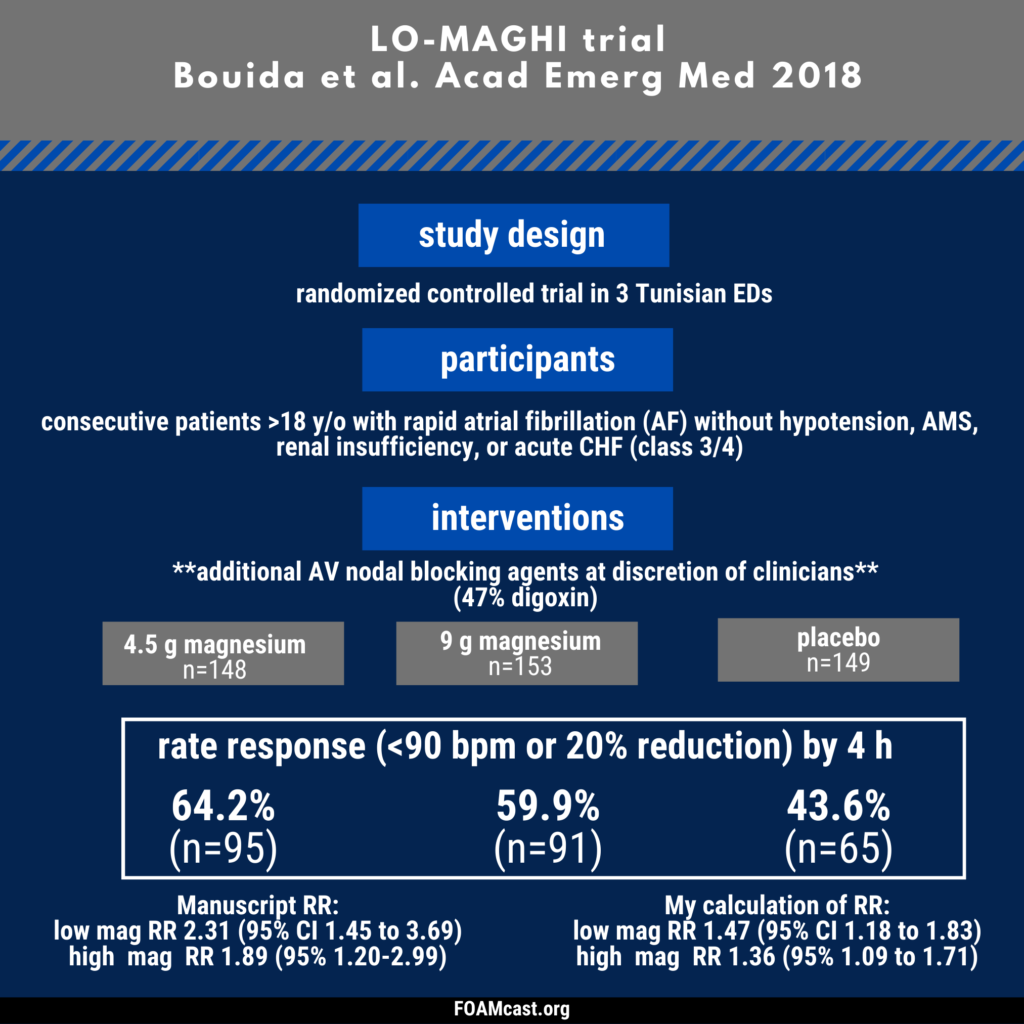
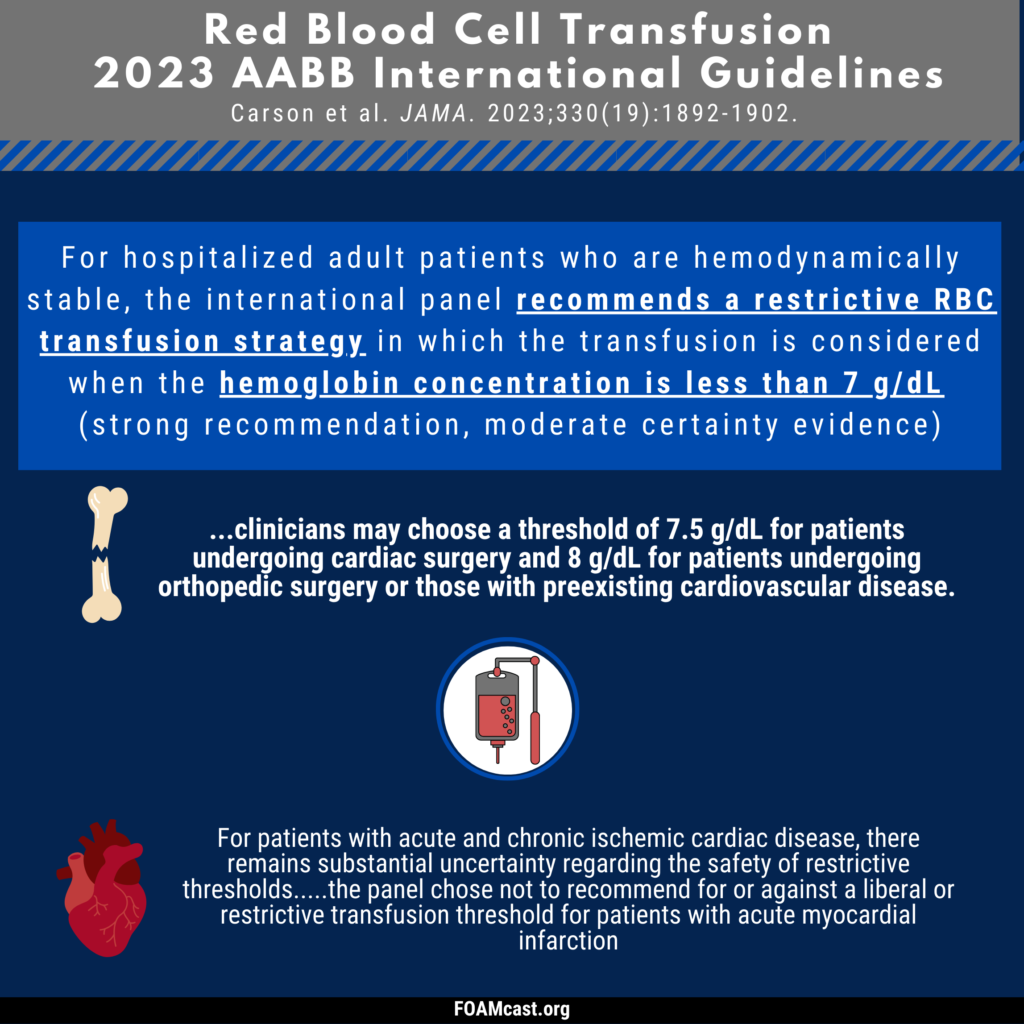
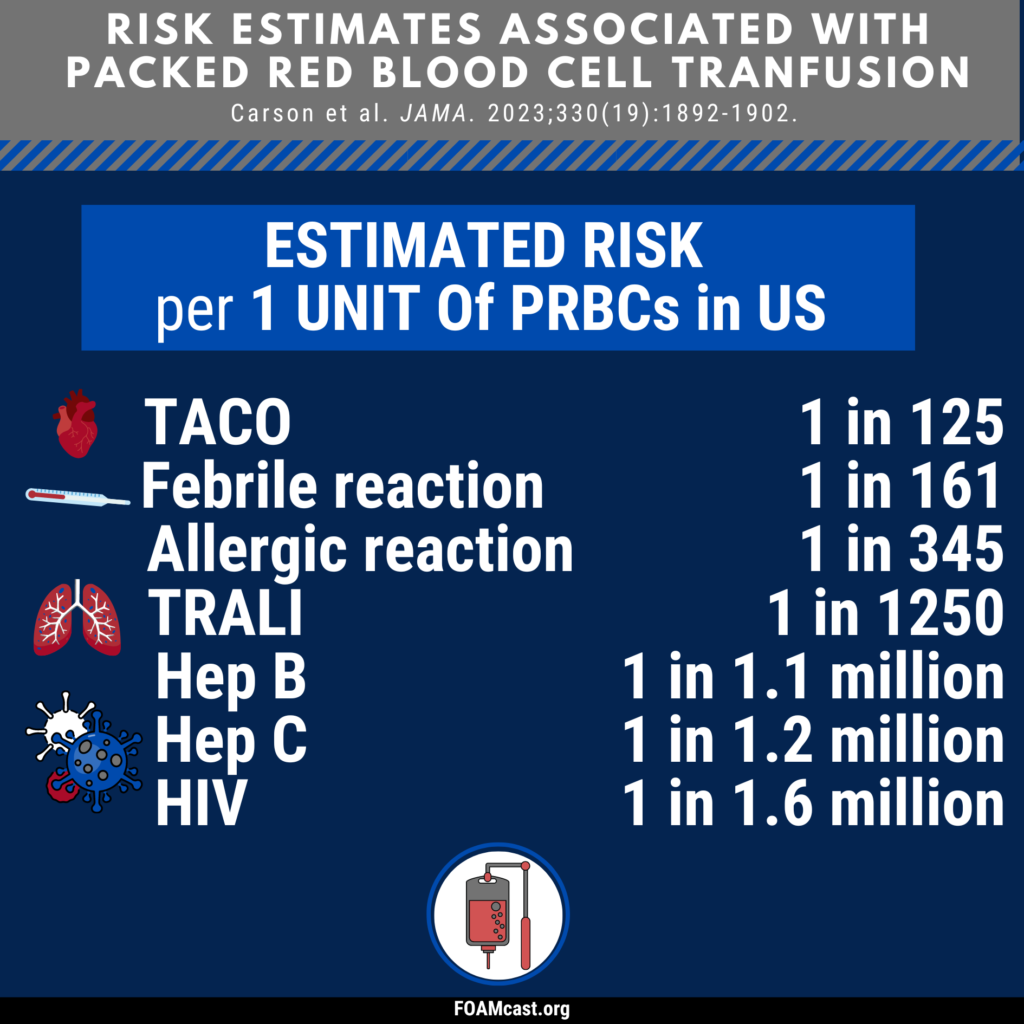
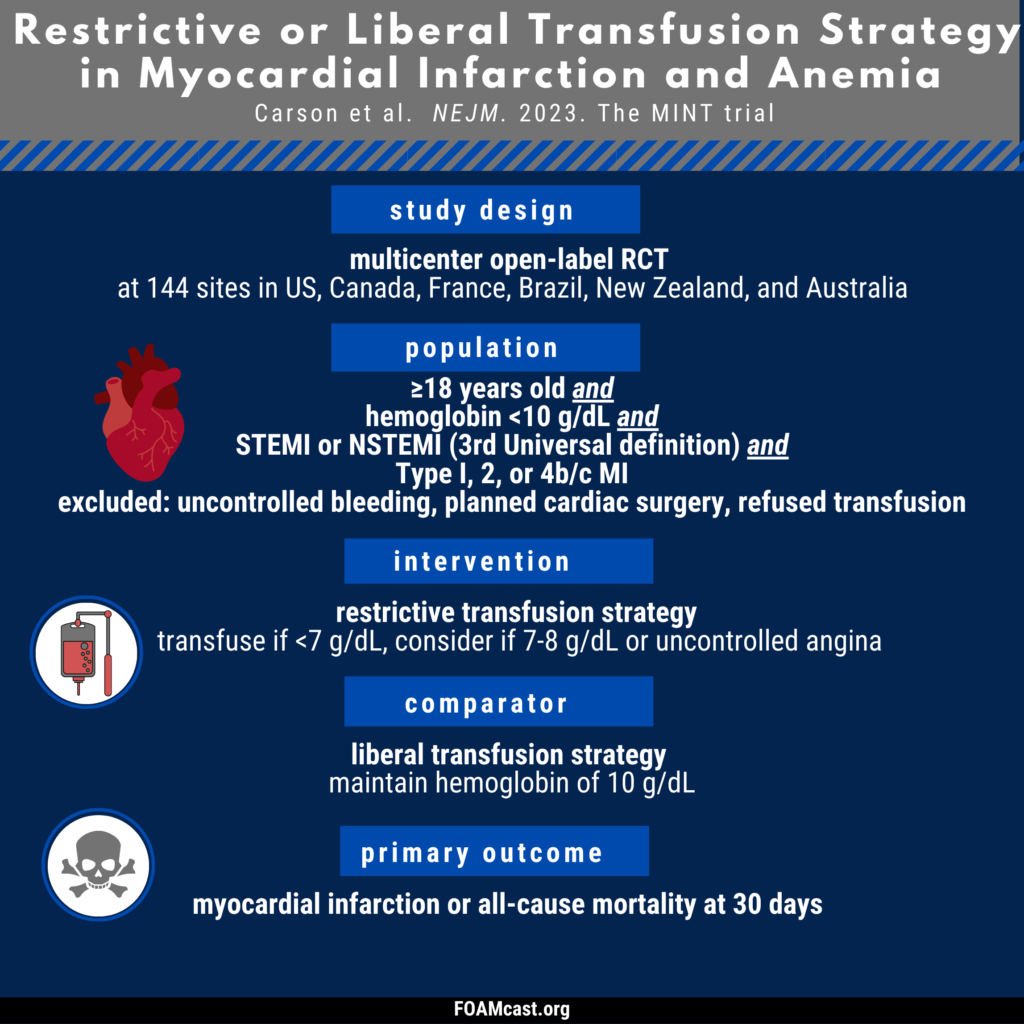
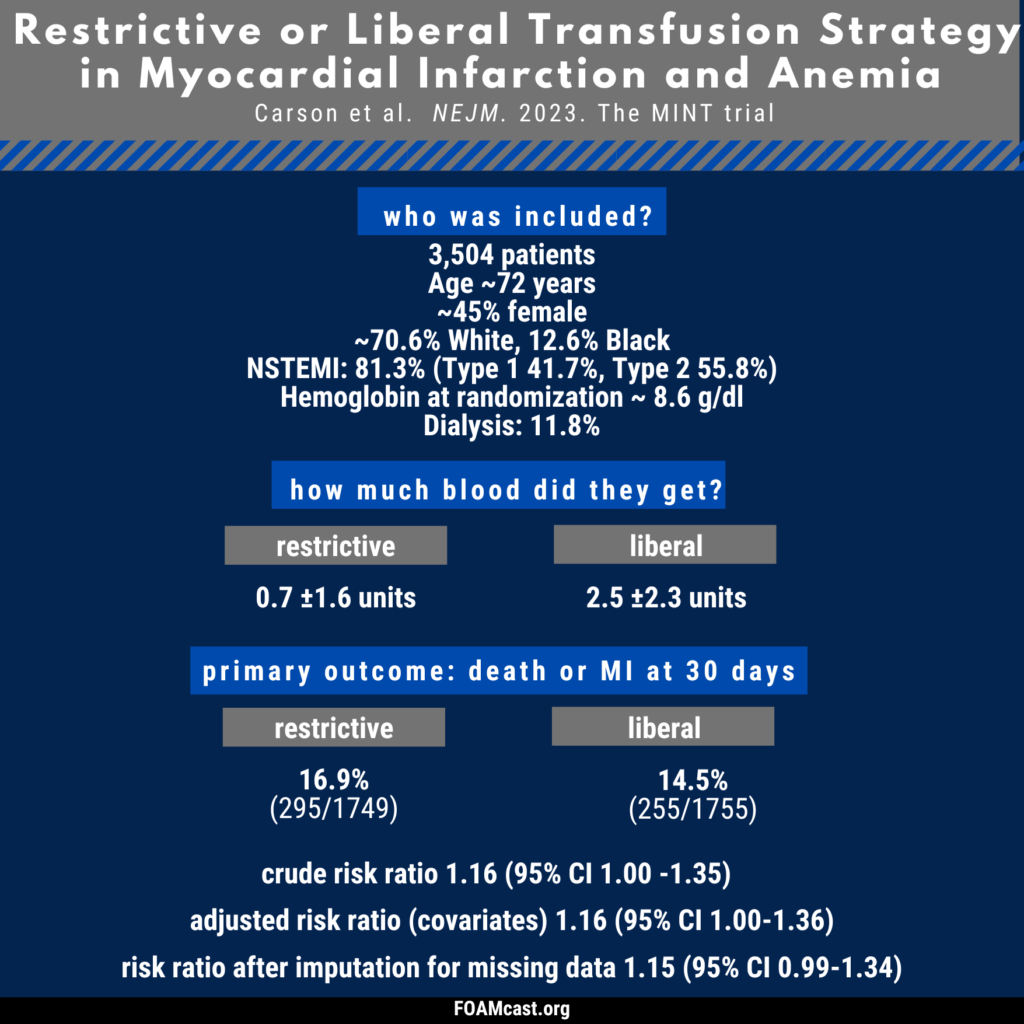
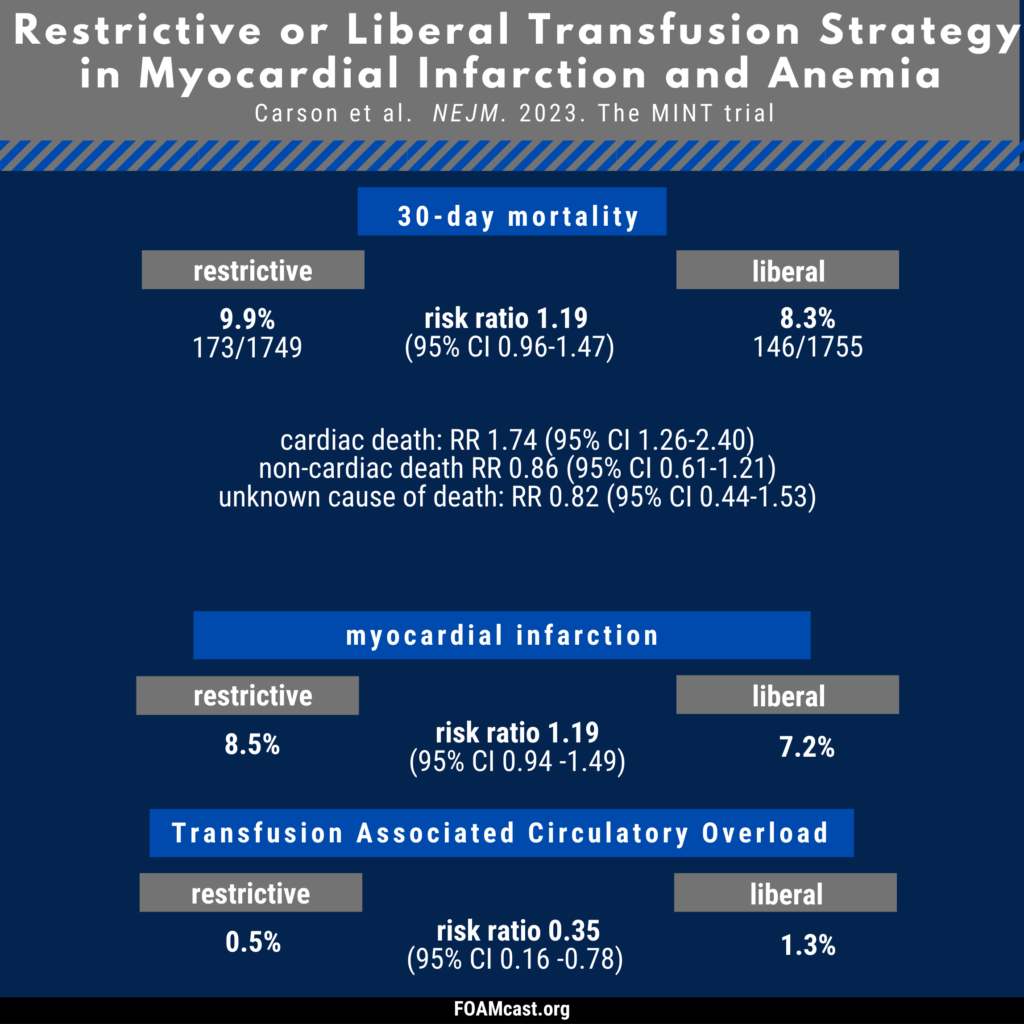
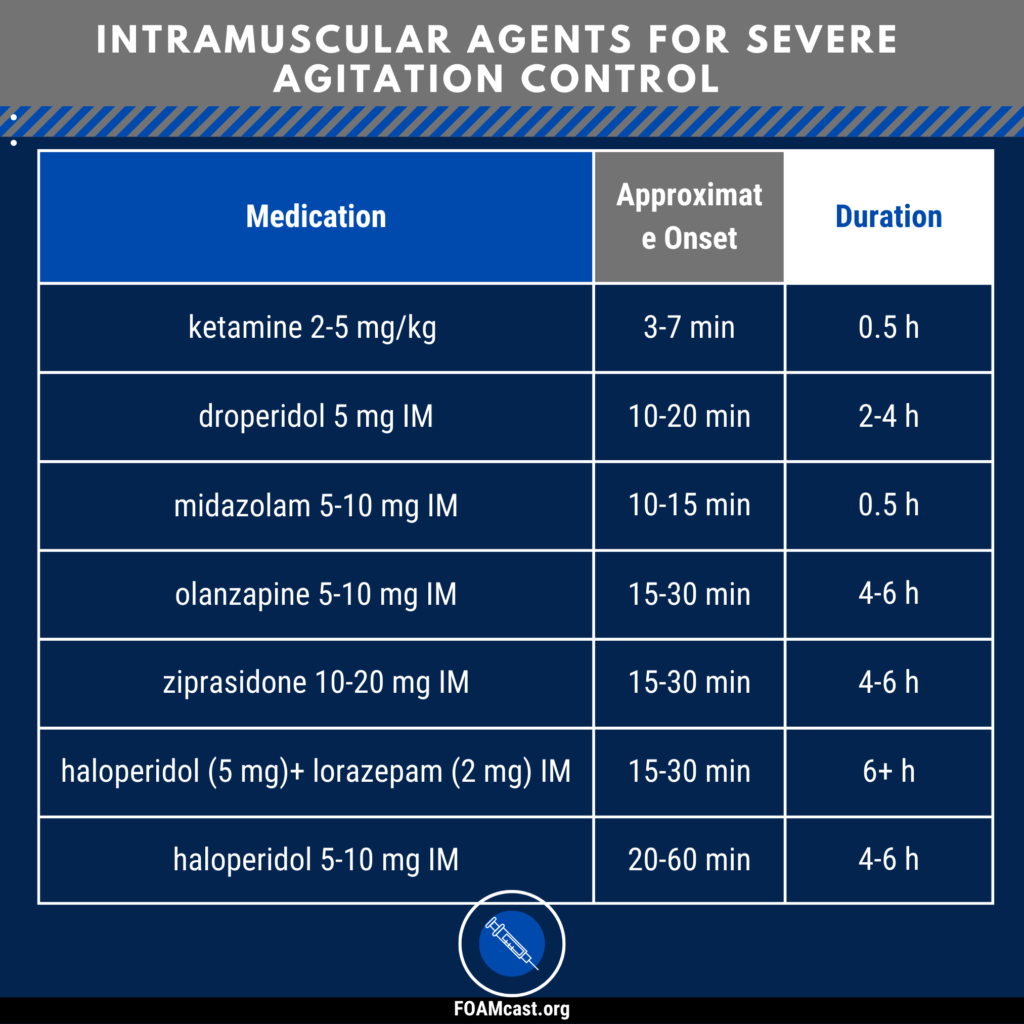
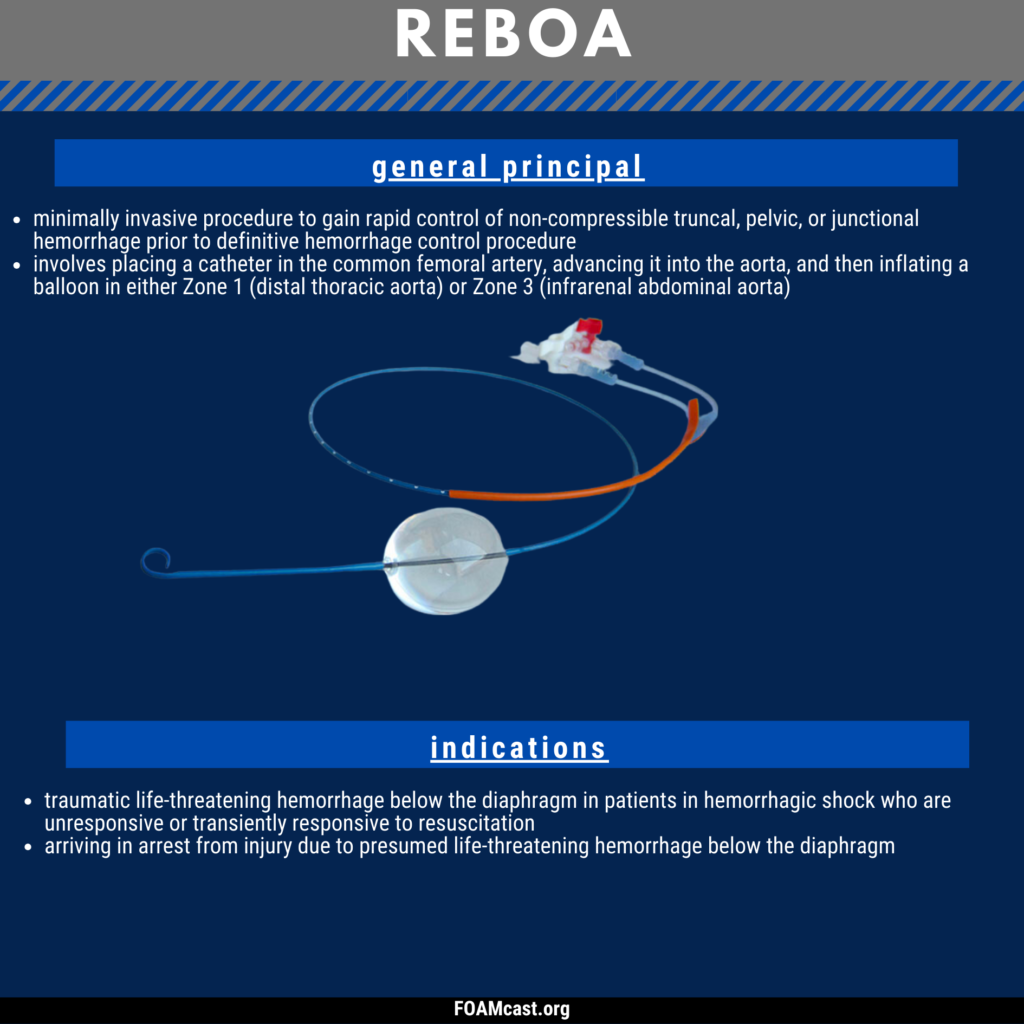
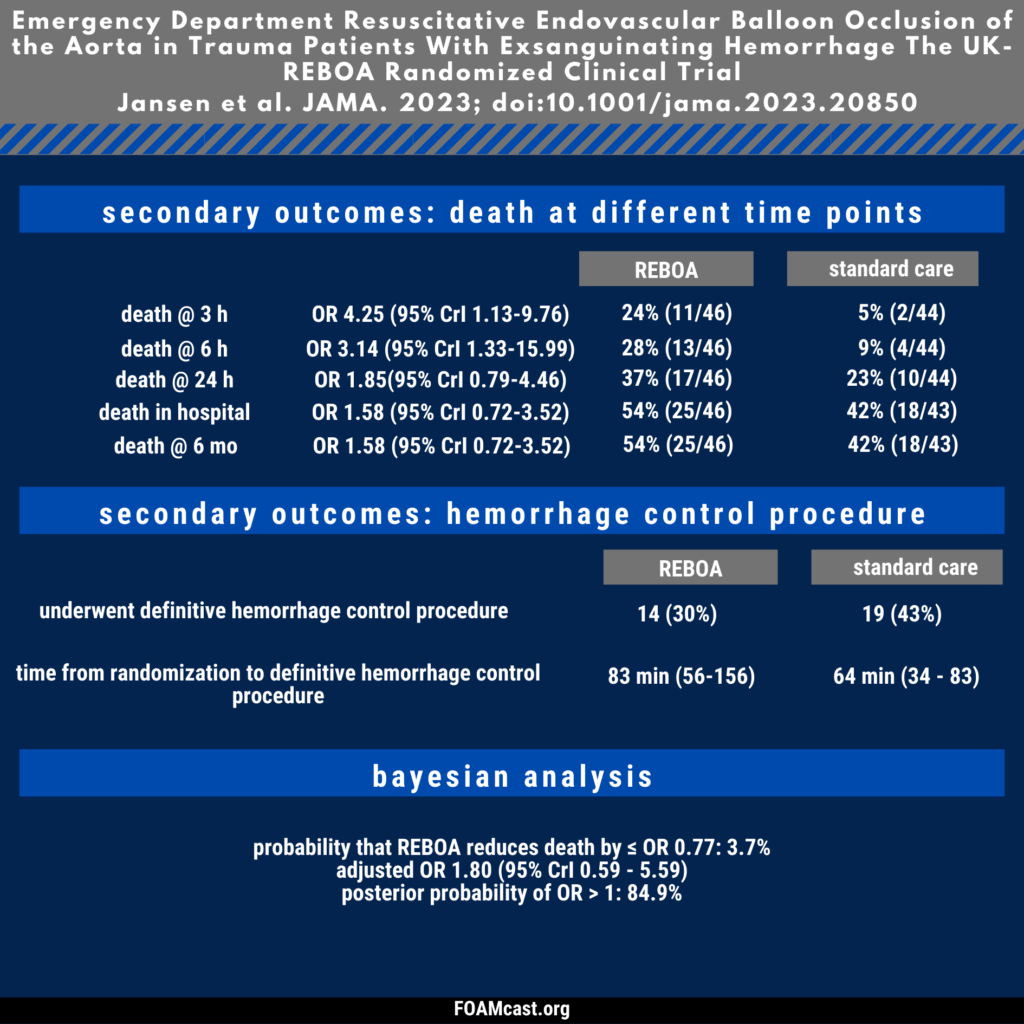
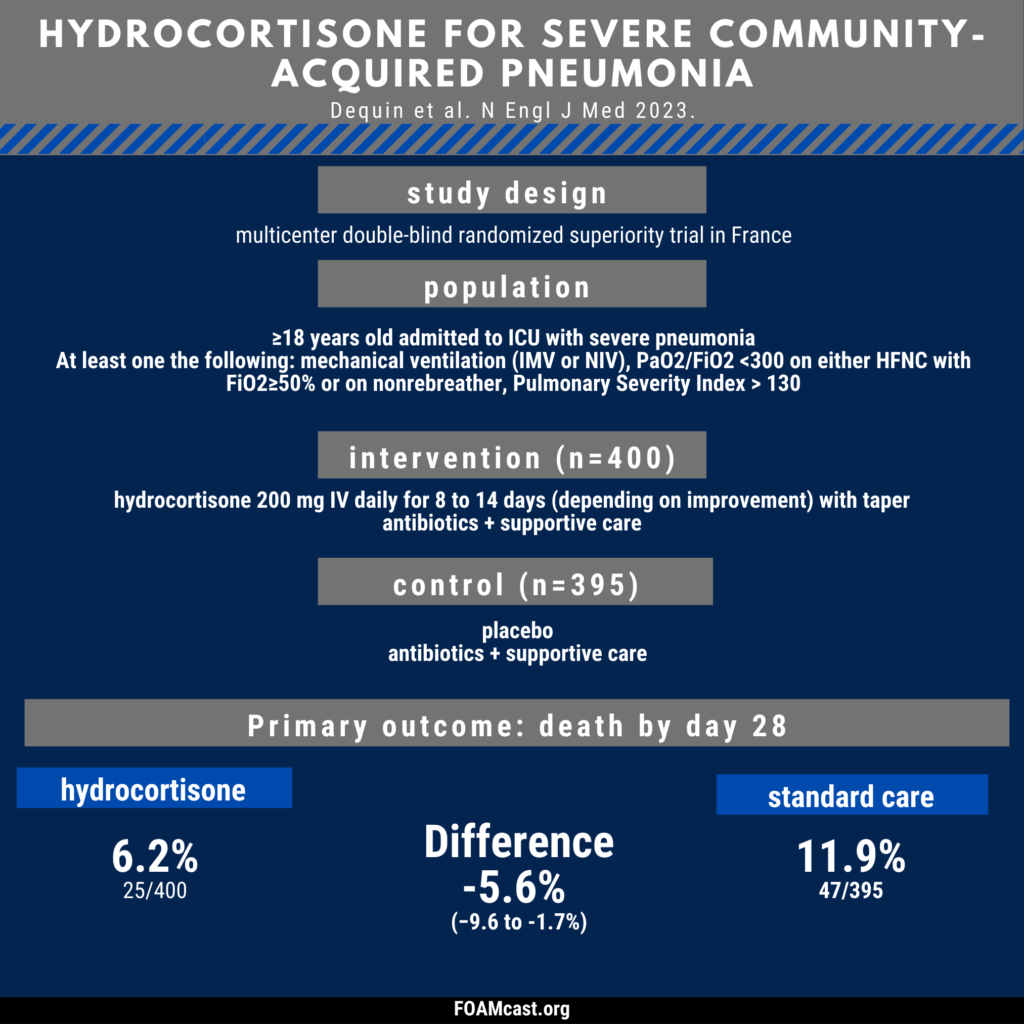
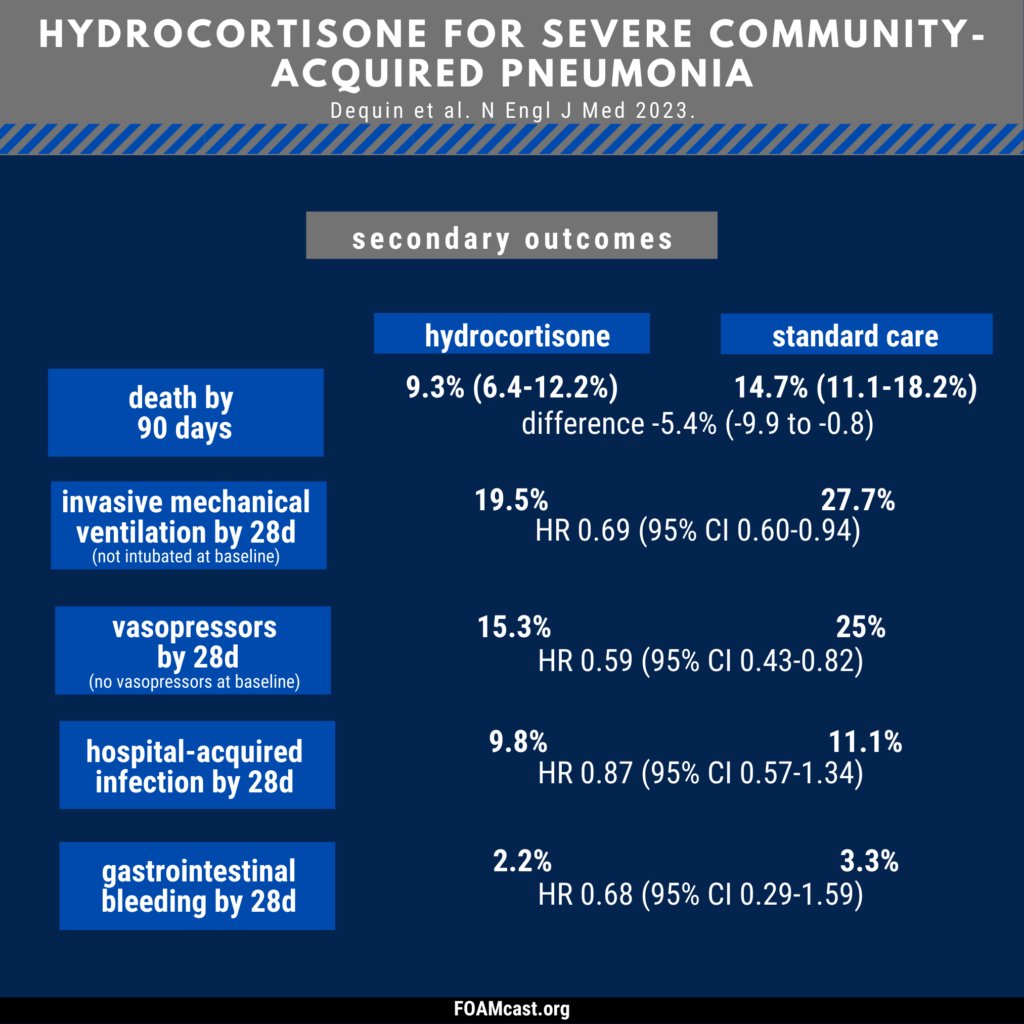
Apple Podcasts, Spotify, Listen Here
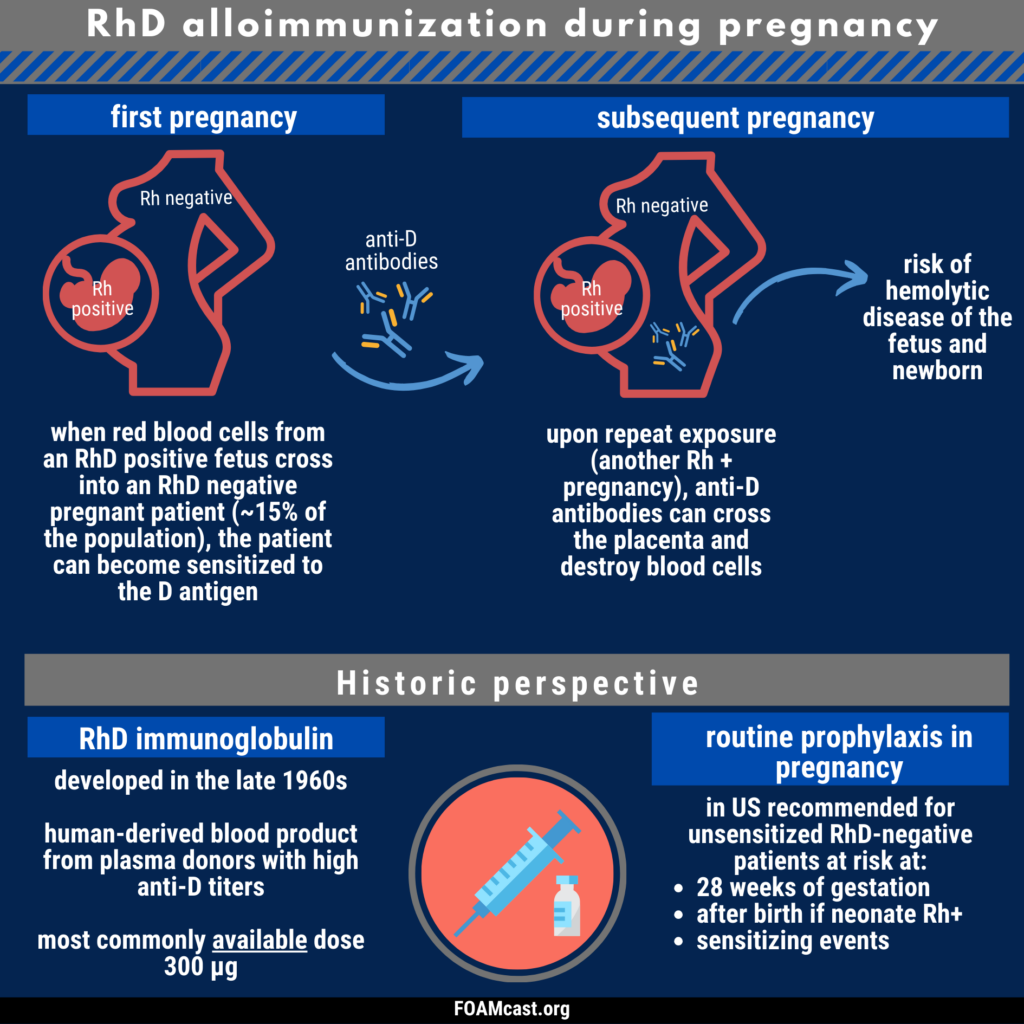
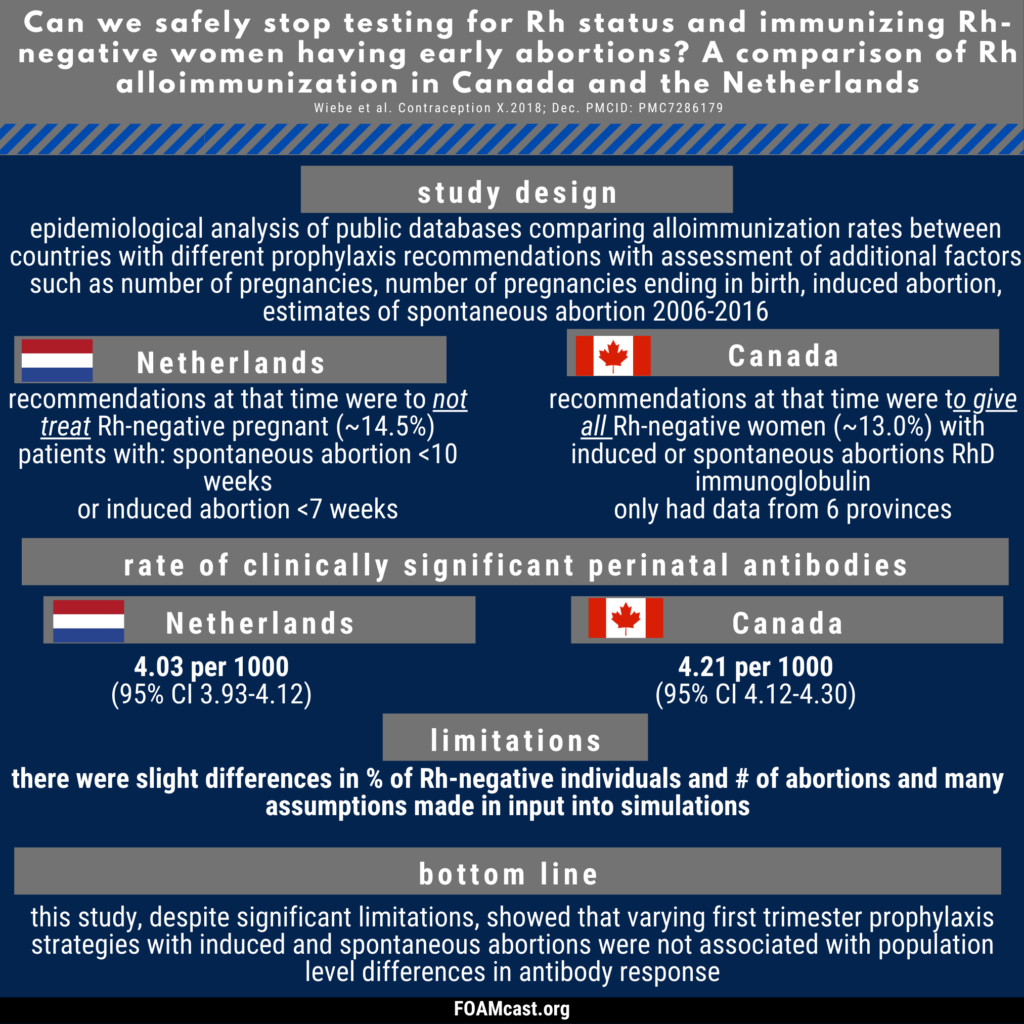
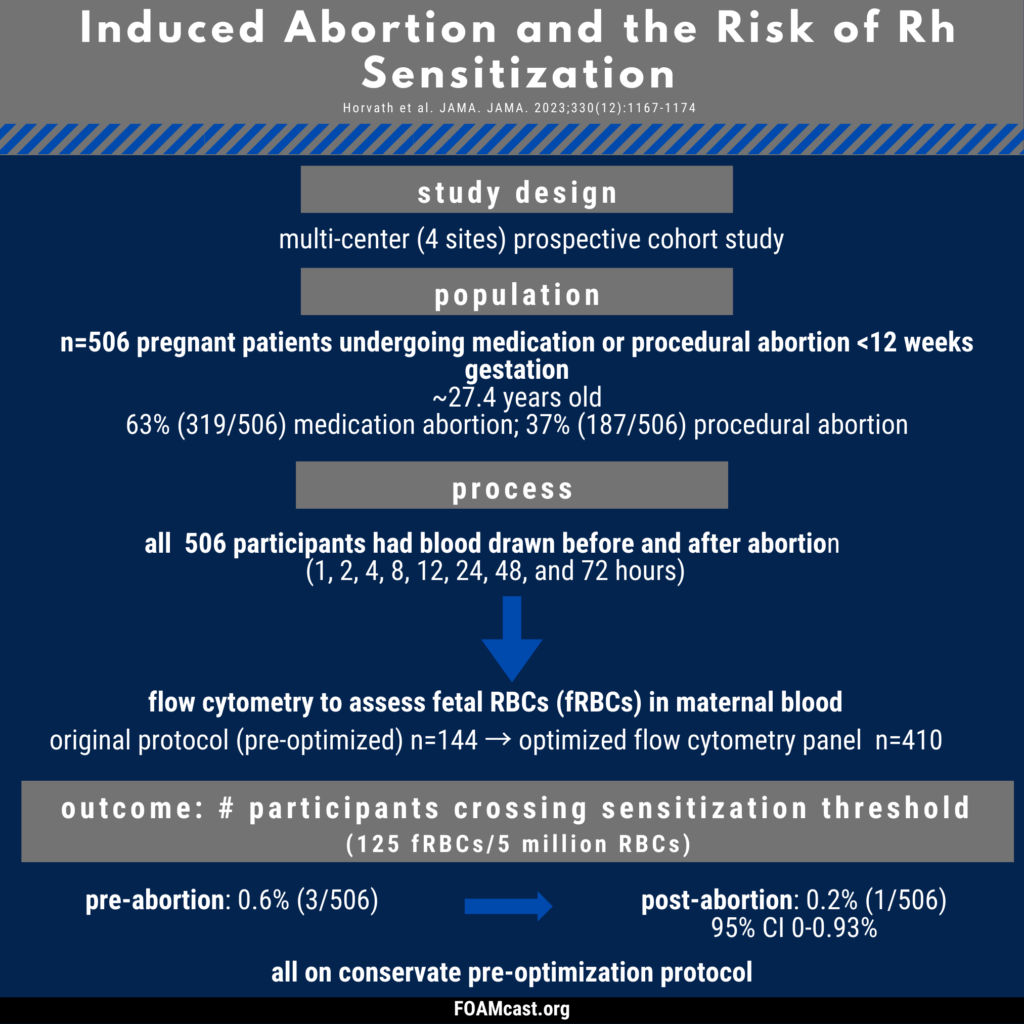
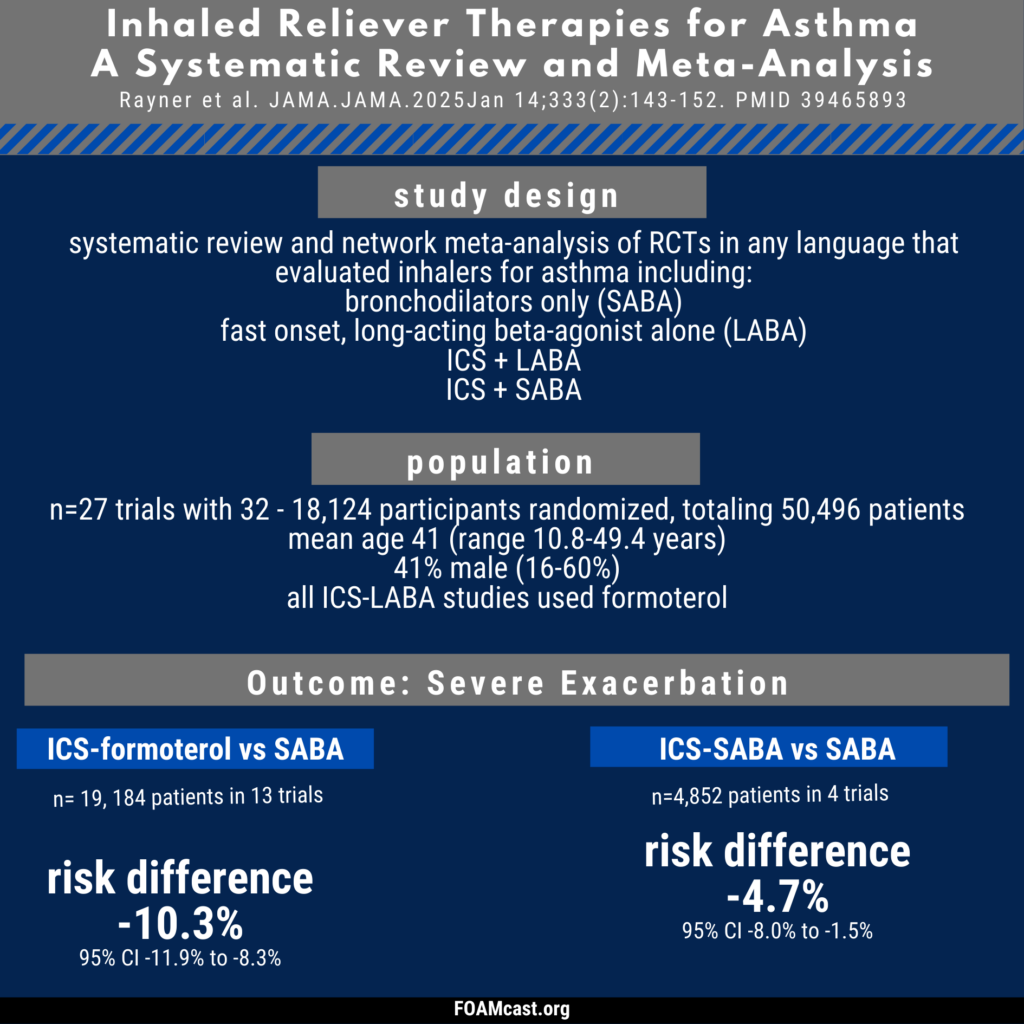
Historically, care of patients in the emergency department (ED) in the first trimester with any kind of abortion (e.g. spontaneous “miscarriage”) underwent blood type and Rh testing followed by RhD immunoglobulin prophylaxis if Rh+. However, many guidelines now recommend foregoing this process in first trimester abortion (spontaneous or induced). In this episode, we dive into the evidence behind these varying recommendations.