Despite multiple Phase II/III vaccine trials of >30,000 participants, pregnant patients or those who are lactating have been largely excluded. In this podcast, we cover what we know (and what we don’t know thus far). The Pfizer BNT162b2 trial data submitted for FDA Emergency Use Authorization does include information on a minuscule number of patients who were immunized and became pregnant, as does the Moderna briefing. Although the risk of the vaccine in pregnancy is thought to be very low, the decision to receive the vaccine during pregnancy should balance the risks of the pregnant individual (to their health/family etc) and their comfort. At the University of Massachusetts Medical School – Baystate, we created a decision aid to help.

The biggest potential risk appears to stem from the reactogenicity of the vaccine – specifically the development of fever. However, the evidence on the harms from maternal fever during pregnancy is variable. Regardless, individuals who are pregnant and receive the vaccine, should probably take acetaminophen if they develop fever.

The Society for Maternal and Fetal Medicine has released statements on vaccines in SARS-CoV-2.

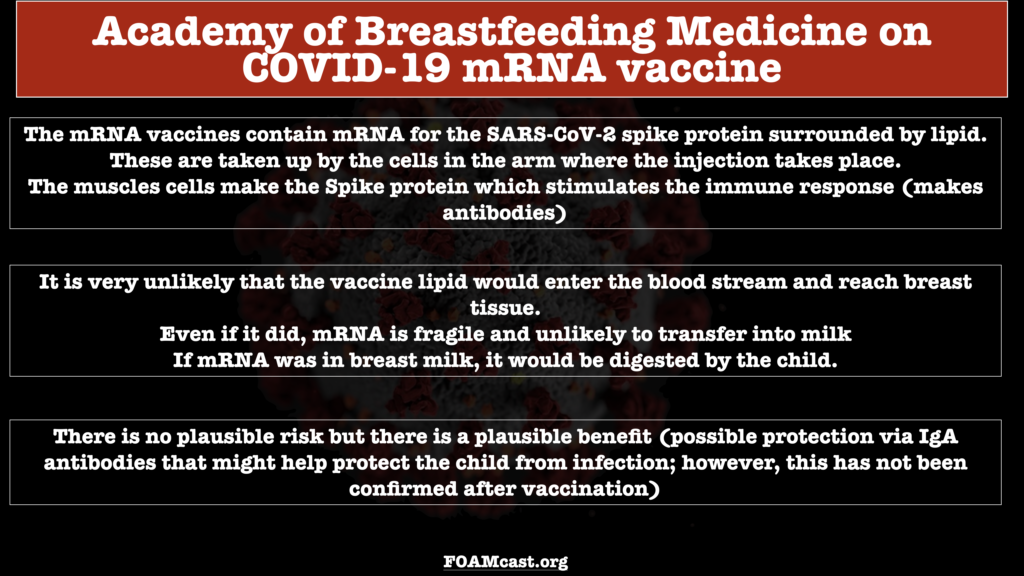
In the United Kingdom, Public Health England has released guidance for their population on vaccination in pregnancy and breastfeeding which are rooted in the lack of data

One thought on “SARS-CoV-2 Vaccine in Pregnancy / Lactation”
Comments are closed.