Apple Podcasts , Spotify, Listen Here
On September 24, 2021, the United States’ CDC made recommendations on who should or may receive a COVID-19 vaccine booster. These recommendations overstepped the vote made by the CDC’s Advisory Committee on Immunization Practices (ACIP), who voted against the recommendation that those 18-64 who have occupational exposure may receive a booster vaccine. In this podcast, we discuss the current evidence as of September 24, 2021 on boosters as well as the evidence-based risk-benefit analysis from the CDC ACIP meeting. Jeremy’s insights can be found at Inside Medicine.
First, the data presented from Pfizer is minimal and involves a total of 312 individuals who received boosters. We have no insight into effectiveness based on this.

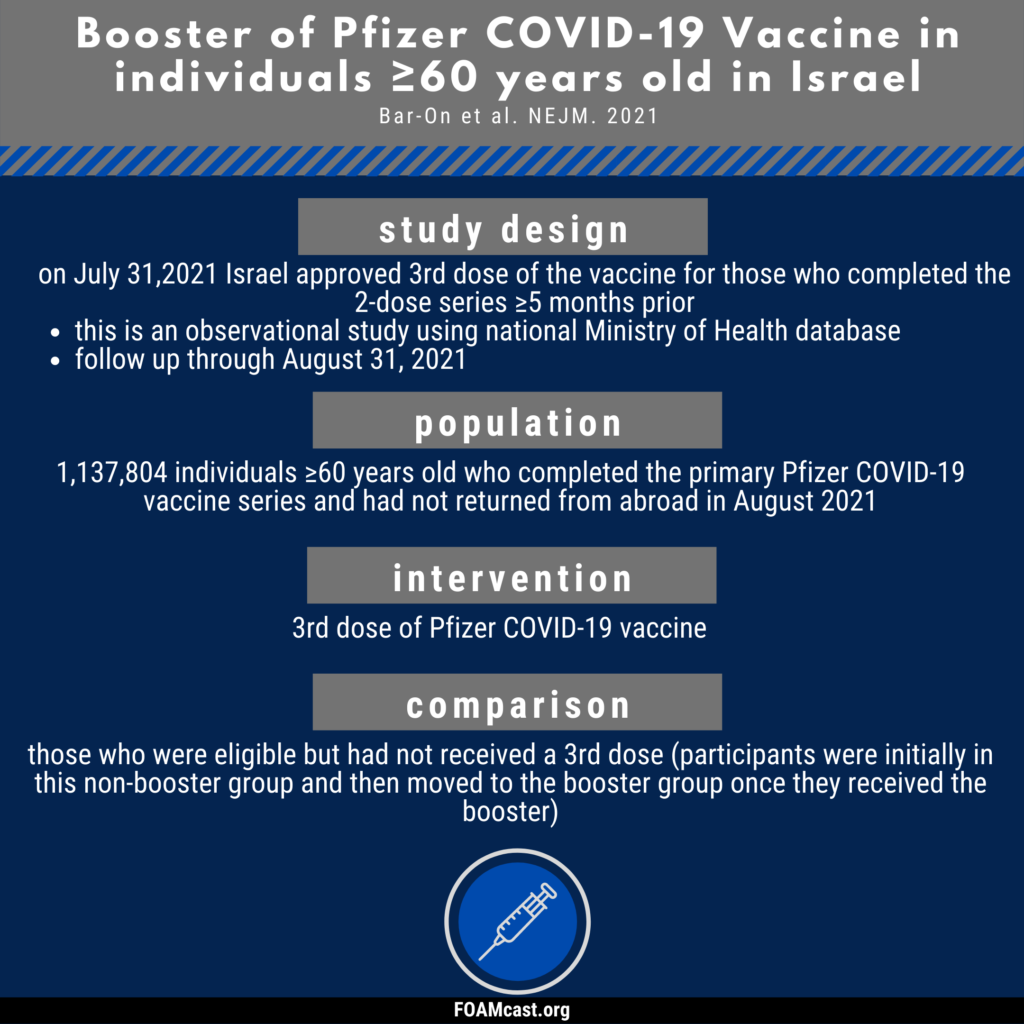
In Israel, boosters were available to those ≥60 who completed the series ≥5 months prior beginning at the end of July. Bar-On et al report on this natural experiment.

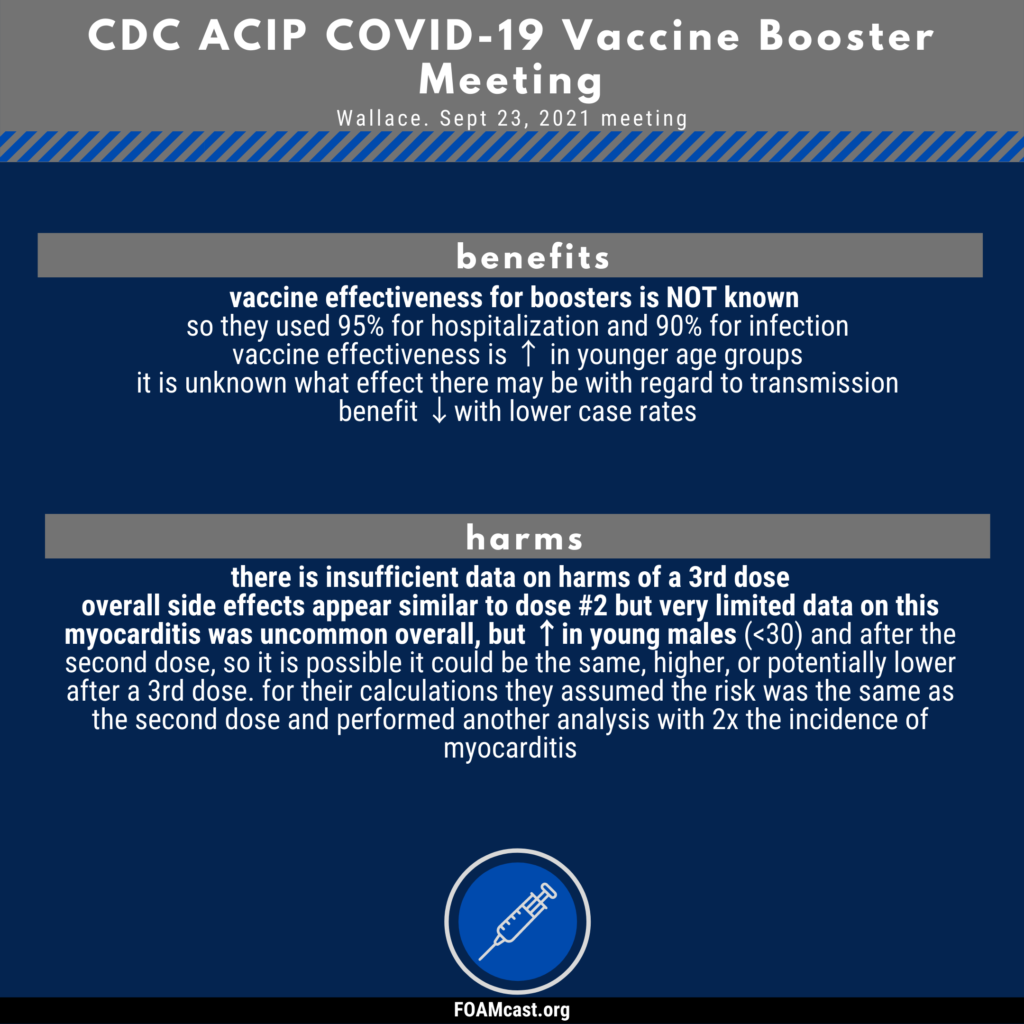
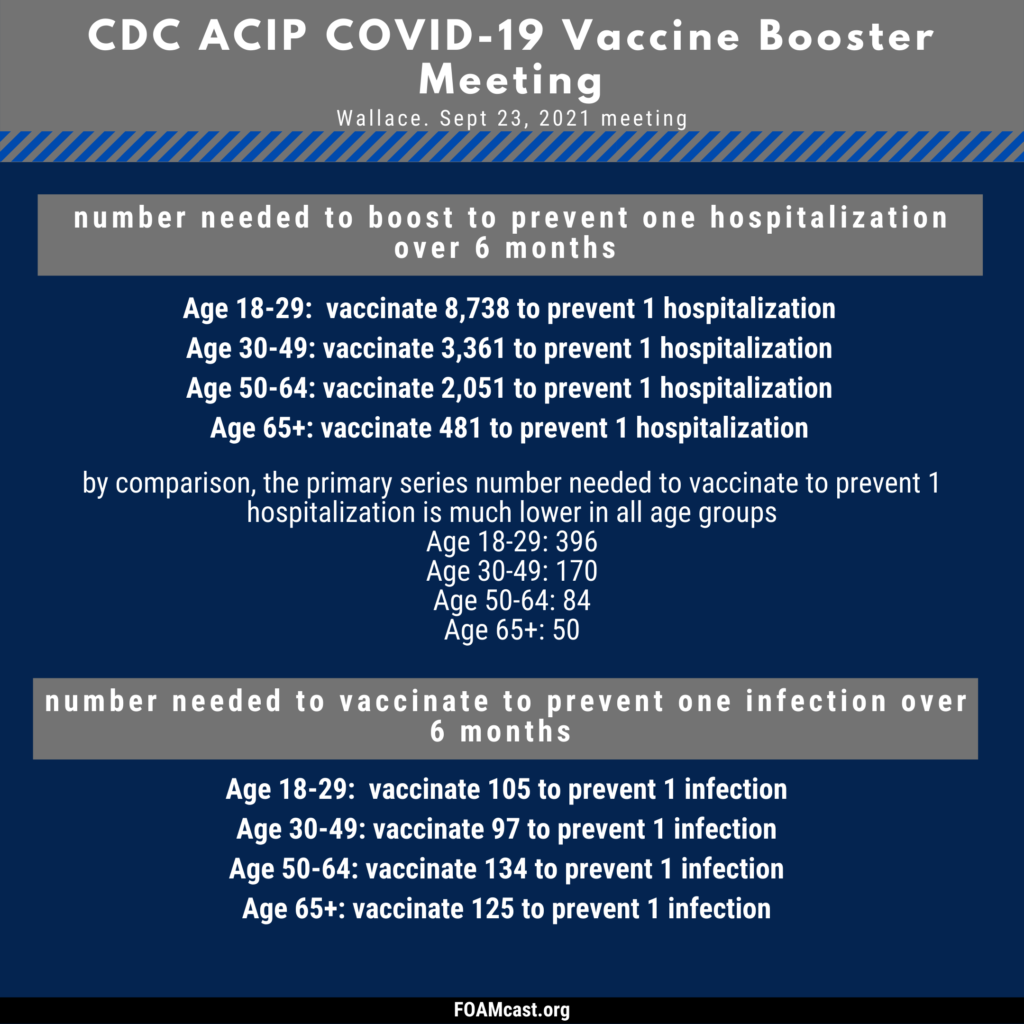
The data presented at the CDC ACIP meeting can be found in the presentation slides available here.